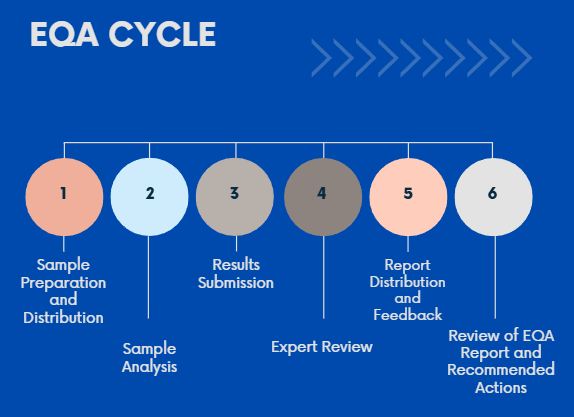
External Quality Assessment (EQA) is a procedure for the systematic assessment of the quality of analysis where unknown samples are tested at regular intervals.
EQA aims to ensure that test results are reliable and comparable no matter where they are performed. Quality assurance of clinical tests is crucial for patient safety.
All hospital laboratories participate in EQA, and point-of-care testing sites are recommended to participate in EQA schemes by
Irish National Near Patient Testing Guidelines
HSE Outline Strategic Plan for Laboratory Services 2026 – 2035

